LIFTCHEM-UV Curing Raw Materials, Pharmaceutical Intermediates
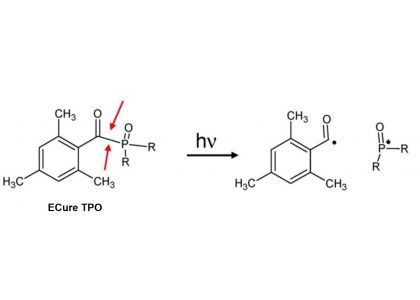
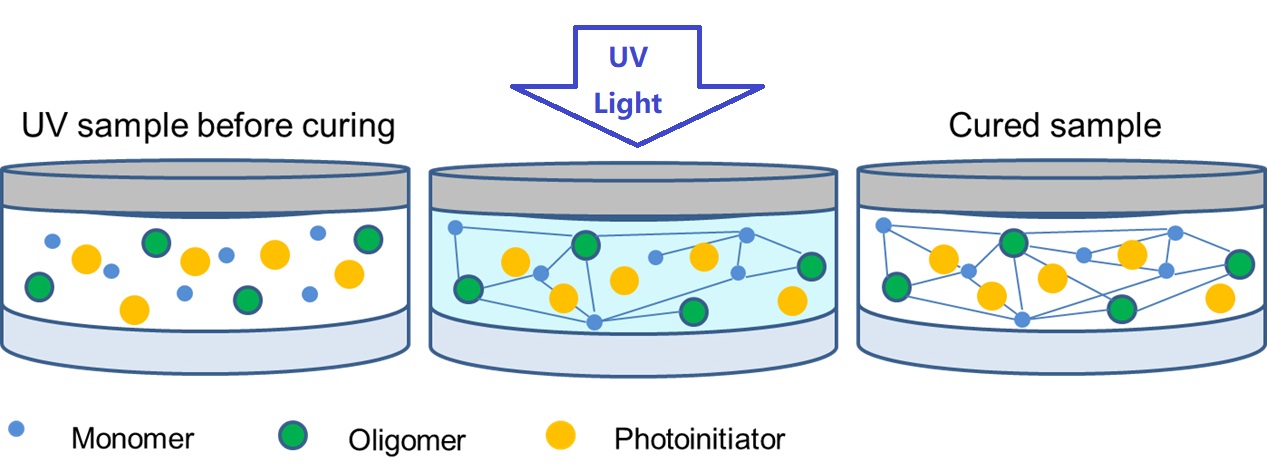
Photoinitiator, also known as Photosensitizer or Photocuring Agent, is a kind of energy that can absorb a certain wavelength in the ultraviolet region (250~420nm) or visible region (400~800nm). Produce free radicals, cations, etc., resulting in monomer polymerization of cross-linked solidified compounds. Photoinitiator is the key component of photocurable materials, which plays a decisive role in the photocuring speed of photocurable materials. They usually have the following properties:
Photosensitivity: The ability to absorb specific wavelengths of light, through light excitation to obtain a high energy or excited state.
High chemical activity: photoinitiators in the excited state are able to react with other molecules to produce free radicals or ions and trigger chemical reactions.
Controllability: The activity of the photoinitiator can be regulated by changing the intensity and wavelength of the light source.
In photopolymerization processes, photoinitiators are added to a monomer or pre-polymer formulation. When illuminated with the appropriate light source, the photoinitiator absorbs light energy and generates free radicals. These free radicals react with the monomer molecules, initiating a rapid chain reaction that forms cross-links and transforms the liquid or viscous formulation into a solid, cured material.
Based on their initiation mechanism, chemical structure, and the type of radiation absorbed, photoinitiators are classified as follows:
Photoinitiators can be classified according to their initiation mechanism, chemical structure, type of radiation absorbed, and role in polymerization.
1. Initiation mechanism: photoinitiators can be divided into free radical type, cationic type, anionic type and ring opening translocation polymerization photoinitiators.
2. Chemical structure: Most of the photoinitiators are organic compounds with benzoyl groups, and the common ones are benzoyl derivatives, benzyl derivatives, α-carboxyl ketones, α-amino ketones, phosphonoxides, benzophenones, thioxanthones and other compounds.
3. Absorption radiation type: photoinitiator can be divided into ultraviolet light (250~420nm) initiator and visible light (400~700nm) initiator.
4. Role in polymerization: free radical photoinitiators are divided into cracking type and hydrogen-stealing type, in which cracking type includes benzoin and its derivatives, acetophenones, aromatic ketones and acylphosphine oxides, etc., while hydrogen-stealing type includes benzophenone, thioxanthrone, camphorone and diimidazole. Cationic photoinitiators mainly include aryl diazonium salt, diaryl iodonium salt, triaryl thioonium salt and aryl ferrocene salt.
There are many kinds of photoinitiators, and each photoinitiator has its unique properties and application fields. When selecting a photoinitiator, it is necessary to comprehensively consider the specific application requirements, light source conditions and the compatibility of the system. The performance and effect of photoinitiator are also affected by factors such as light intensity, wavelength, temperature, etc., so it is necessary to carry out adequate test and optimization in practical application. If you have any product needs, please feel free to contact us, LIFTCHEM will serve you wholeheartedly.
Photoinitiators have been widely used in many fields, including photolithography, photocuring, photopolymerization, photodegradation, and photocatalysis. In the light curing system, UV adhesive, UV coating, UV ink, etc., after accepting or absorbing external energy, the photoinitiator will decompose into free radicals or cations, which will trigger polymerization. In addition, the photoinitiator is also used in photoresist and supporting chemicals, electronic products, home improvement building materials and medicine and other fields.
The photoinitiator market has developed rapidly in the past few years, and the scale has shown a steady growth trend. With the development of digitalization and intelligence, photoinitiators, as an efficient and intelligent chemical material, will have a broader market prospect. In particular, the increasing demand for applications in many industries is driving the expansion of the market size.
In terms of development trends, the research of photoinitiators is developing in the direction of being more efficient, more environmentally friendly, and more adaptable to specific needs. New photoinitiators are constantly being developed to meet the needs of different industries and applications. In addition, with the development of science and technology, the application field of photoinitiators is also expanding, providing more possibilities for future market growth.
In general, photoinitiators are an important class of chemical substances with a wide range of applications and huge market potential. With the advancement of science and technology and the broadening of application fields, the development prospects of photoinitiators will be broader.